How Do You Find The Mass Of A Carbon Atom: Unveiling Atomic Secrets
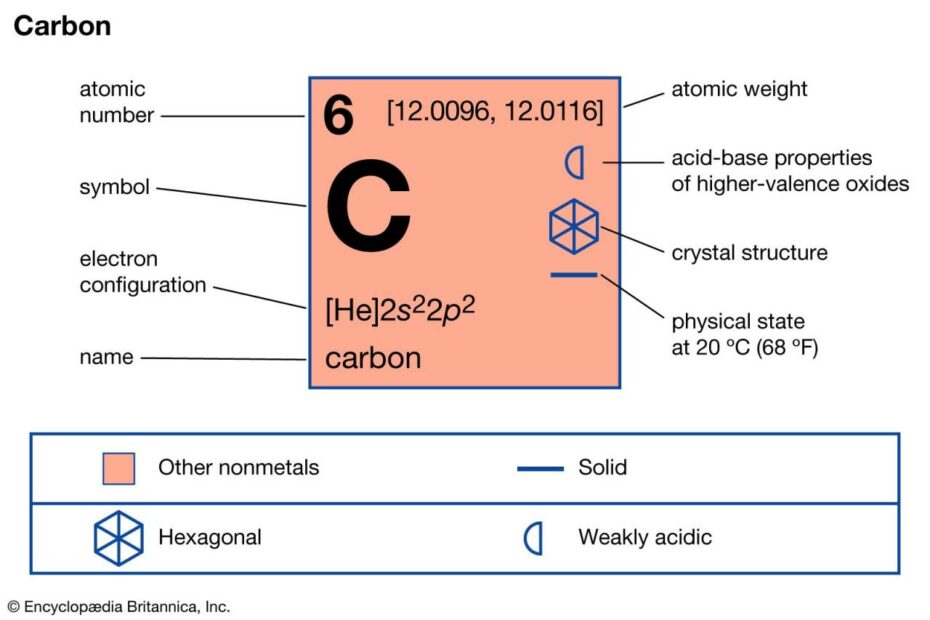
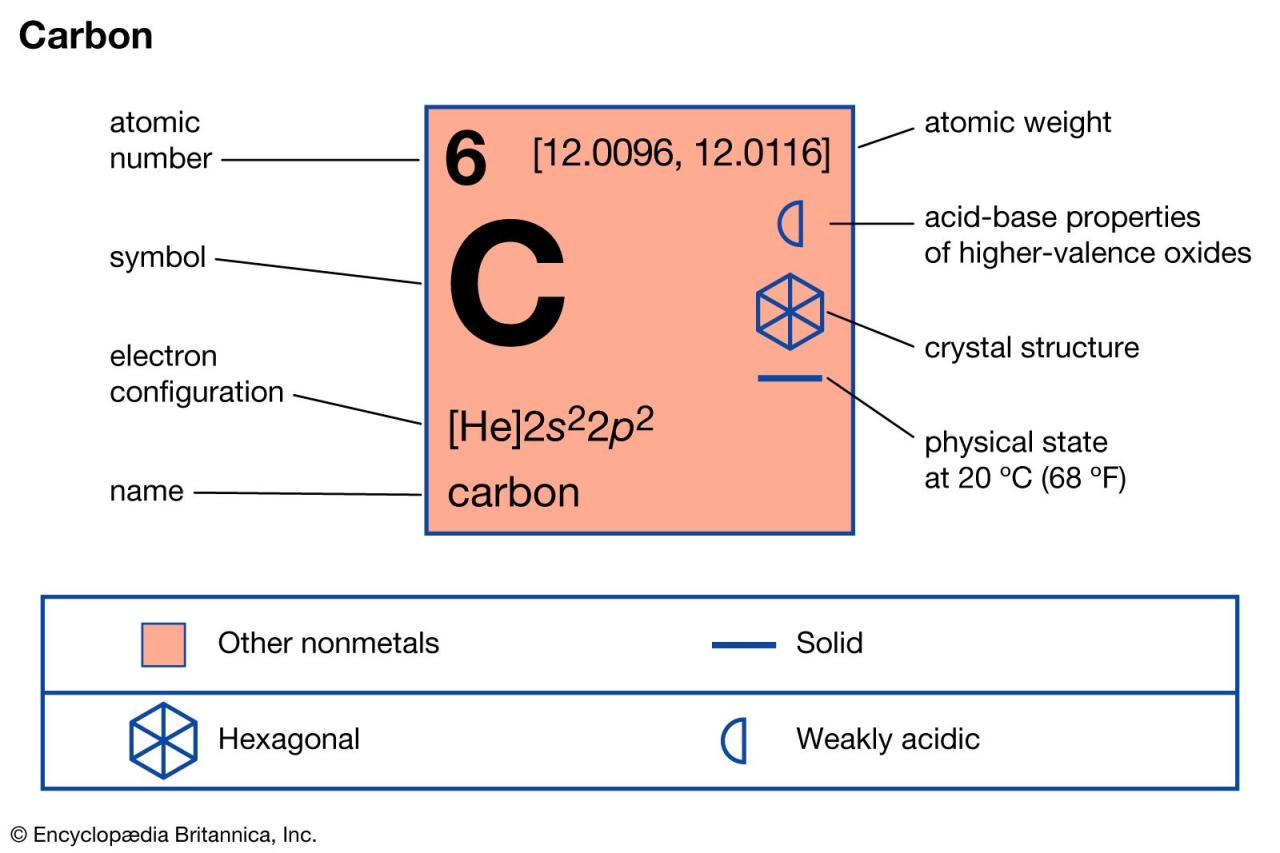
How To Find The Mass Of One Atom Of Carbon (C)
Keywords searched by users: How do you find the mass of a carbon atom what is the mass of 1 atom of carbon in grams, mass of 1 carbon atom, mass of one carbon atom in amu, mass of one carbon atom in kg, mass of carbon-12 atom in grams, mass of one atom of carbon 12, 1 gram atom of carbon contains how many moles, mass of carbon-12 atom in kg
How Do You Find The Mass Of One Carbon Atom?
“How can we determine the mass of a single carbon atom? This question often arises when we delve into the world of atomic structure. In this YouTube video, we’ll explore the process of finding the mass of one atom of carbon (C) and understand the essential factors involved in this calculation.
To calculate the mass of one carbon atom, we need to consider the concept of moles. Moles serve as a bridge between atoms and grams, allowing us to make this determination accurately. By manipulating the units and utilizing the mole concept, we can arrive at the desired answer.
In the video, we will demonstrate the step-by-step process, ensuring that moles are appropriately utilized in both the numerator and denominator of our calculations, ultimately canceling each other out to leave us with the mass in grams. When correctly executed, this calculation should yield a result of approximately 2.0 x 10^23 grams, providing us with the mass of one individual carbon atom.”
What Is The Mass Of The Carbon Atom?
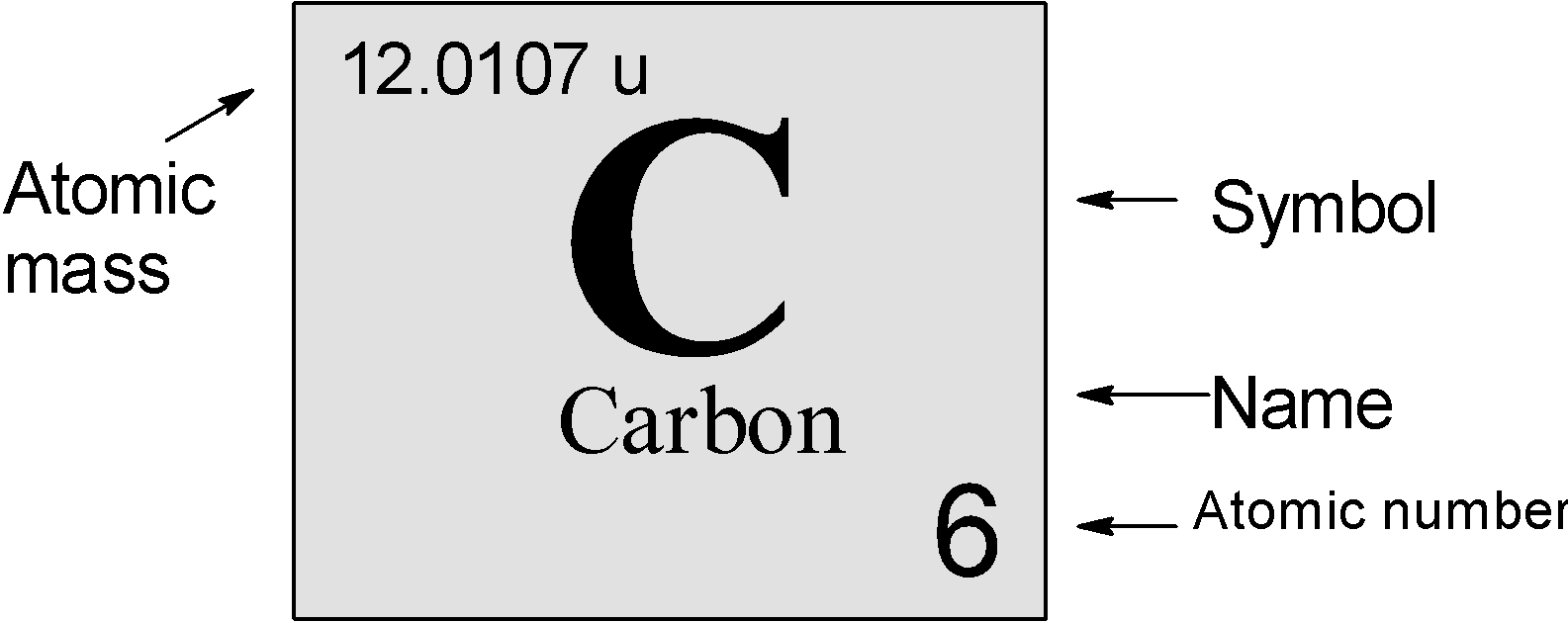
What is the mass of a single carbon atom? To answer this question, let’s first establish that the mass of one mole of carbon atoms is 12 grams. Now, we want to determine the mass of an individual carbon atom. To do this, we can use Avogadro’s number, which tells us that there are approximately 6.022 x 10^23 atoms in one mole of any element. Therefore, to find the mass of a single carbon atom, we divide the mass of one mole (12 grams) by Avogadro’s number.
Mass of 1 atom of carbon = (Mass of 1 mole of carbon) / (Avogadro’s number)
Mass of 1 atom of carbon = 12 grams / 6.022 x 10^23 atoms ≈ 1.99 x 10^-23 grams.
So, the mass of one atom of carbon is approximately 1.99 x 10^-23 grams.
Aggregate 26 How do you find the mass of a carbon atom


Categories: Found 57 How Do You Find The Mass Of A Carbon Atom
See more here: kotop.shinbroadband.com

To calculate the mass of a single atom of carbon, we just need to divide the molar mass of 12.0 g (0,012 kg) by the number of particles per mole (Avogadro’s number). Doing so yields 1.99 ´ 10–26 kg as the mass of a carbon atom.Mass of 1 atom of carbon =126.022×1023 = 1.9×10−23g. Q. If one mole of carbon atom weighs 12g, what is the mass in grams of 1 atom of carbon?For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called the mass number. This is because each proton and each neutron weigh one atomic mass unit (amu). By adding together the number of protons and neutrons and multiplying by 1 amu, you can calculate the mass of the atom.
Learn more about the topic How do you find the mass of a carbon atom.
- The Mass of Atoms and Molecules
- How to Find the Mass of One Atom of Carbon (C) – YouTube
- If one mole of carbon atom weighs 12 g what is the mass of 1 …
- 2.2.5: Average Atomic Mass – Biology LibreTexts
- How did scientists estimate the mass of a carbon atom (in grams … – Quora
- What is the atomic mass unit (AMU or amu) and how is it determined?
See more: https://kotop.shinbroadband.com/real-estate/