Why Is Gold The Least Reactive Metal In The Periodic Table?
Reactivity Series Of Metals | Environmental | Chemistry | Fuseschool
Keywords searched by users: Why is gold the least reactive metal why is gold less reactive than silver, why is gold unreactive, is gold reactive or nonreactive, why is gold stable element, is gold flammable, which element is most reactive with gold fluorine chlorine or bromine, why gold is the noblest of all the metals, why is gold unreactive with oxygen
Is Gold The Least Reactive Metal?
Gold occupies a special place in society due to its exceptional chemical inertness. This remarkable quality stems from its status as the least reactive metal when exposed to atoms or molecules in contact with gases or liquids. This non-reactive nature makes gold a highly sought-after material in various applications, from electronics to jewelry, where its stability and resistance to corrosion are of paramount importance.
What Is The Least Reactive Metal And Why?
Platinum stands out as the least reactive among metals due to its exceptional resistance to corrosion, even under extreme temperatures. This unique property classifies platinum as a noble metal, a group renowned for their remarkable inertness. As a result of its exceptional chemical stability, platinum is frequently discovered in its pure, uncombined form, known as native platinum. This characteristic sets platinum apart from other metals, making it an invaluable material in various industrial and scientific applications.
Share 44 Why is gold the least reactive metal
![Reactivity Series of Metals - Chart [and How to remember] - Teachoo Reactivity Series Of Metals - Chart [And How To Remember] - Teachoo](https://kotop.shinbroadband.com/wp-content/uploads/2023/09/reactivity-series-01.jpg)


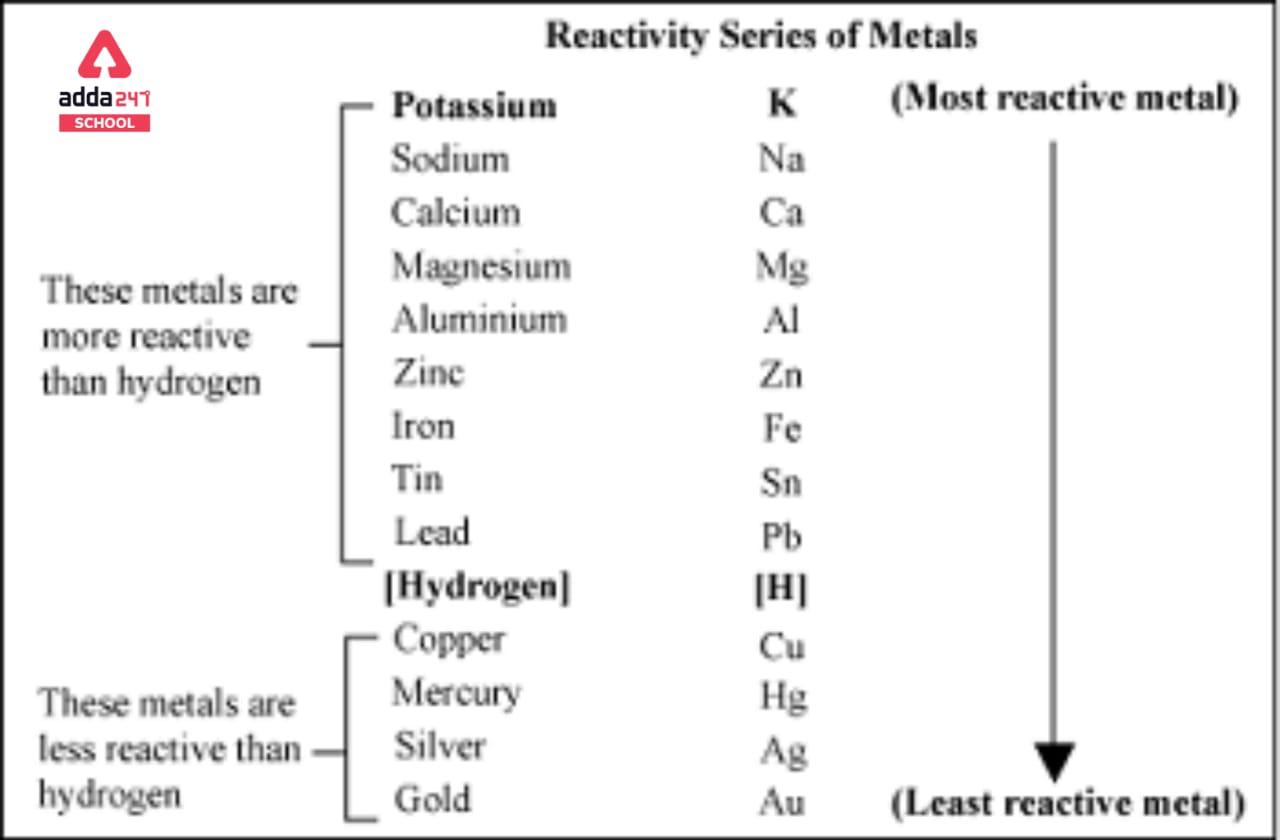
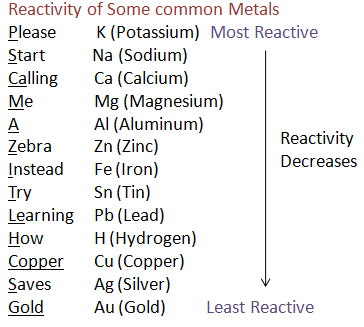

Categories: Collect 12 Why Is Gold The Least Reactive Metal
See more here: kotop.shinbroadband.com

Gold is not very reactive because its unpaired 6s electron is at a lower energy level than its many paired 5p and 5d electrons. So even though gold would like to give up its unpaired electron or accept a partner for it, it usually can’t because of the stability of the higher energy electrons that are already paired.THE unique role that gold plays in society is to a large extent related to the fact that it is the most noble of all metals: it is the least reactive metal towards atoms or molecules at the interface with a gas or a liquid.Platinum is the least reactive metal. It has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. Consequently, platinum is often found chemically uncombined as native platinum.
Learn more about the topic Why is gold the least reactive metal.
- Why isn’t the element gold reactive? – Quora
- Why gold is the noblest of all the metals – Nature
- Which of the following is the least reactive metal? – Toppr
- Gold – why is it so valuable? – Science blog
- Silver and gold react with oxygen at high temperatures.(A) True(B) False
- Gold – Wikipedia
See more: https://kotop.shinbroadband.com/real-estate
![Reactivity Series Of Metals - Chart [And How To Remember] - Teachoo](https://kotop.shinbroadband.com/wp-content/uploads/2023/09/reactivity-series-01-930x620.jpg)